Transthyretin amyloid cardiomyopathy (ATTR-CM) is a rare and progressive disease characterized by misfolded transthyretin (TTR) protein accumulating in the heart muscle and other parts of the body and causing damage.
There is currently no cure for ATTR-CM and treatment focuses on stopping the buildup of misfolded proteins and slowing the progression of the disease.
Treatment of symptomatic disease
Some treatments can help manage the symptoms of ATTR-CM. These include medications that aim to prevent conduction and rhythm problems and maintain higher heart rates.
For example, loop diuretics can help reduce heart and peripheral congestion and lung edema, thereby relieving shortness of breath. These are usually taken with aldosterone antagonists to prevent low potassium levels.
It is important to note that standard heart failure medications such as calcium channel blockers and digoxin are not suitable for patients with ATTR-CM. Drugs like renin-angiotensin system inhibitors and beta-adrenoreceptor blockers are not well-tolerated by patients with ATTR-CM.
Disease-modifying treatments
Disease-modifying treatments aim to prevent the synthesis of TTR, stabilize the structure of the TTR protein or remove misfolded TTR proteins from the body.
They include the TTR protein stabilizers tafamidis (marketed under the brand name Vyndamax or Vyndaqel), acoramidis (marketed under the brand name Attruby), tolcapone (marketed as Tasmar) and diflunisal (under the brand name Dolobid).
There are also TTR tetramer silencers: patisiran, with the brand name Onpattro, vutrisiran marketed as Amvuttra, and eplontersen marketed under the brand name Wainua. The TTR protein is normally found as a tetramer, i.e., a group of four units. These treatments work by inhibiting the synthesis of the TTR protein and, therefore, the formation of the TTR tetramer.
Of these therapies, only tafamidis, acoramidis and vutrisiran have been approved specifically for treating ATTR-CM. The others may be used off-label for the treatment of the disease. Off-label use is using a drug approved by the US Food and Drug Administration for an unapproved use. Patisiran and eplontersen have been approved for the treatment of ATTR with polyneuropathy, or ATTR-PN.
Medical devices
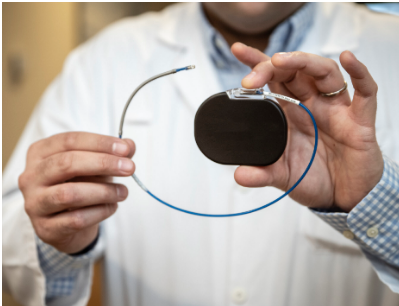
Medical devices are also used to treat patients with ATTR-CM.
These can include permanent pacemakers against heart block and implantable cardioverter-defibrillators for patients with ventricular tachycardia. However, these should only be used for secondary prevention of cardiac amyloidosis.
A left ventricular assist device, which helps the lower left chamber or ventricle of the heart to pump blood to the rest of the body, may also be helpful in some patients with ATTR-CM.
Organ transplantation
In rare cases, patients with ATTR-CM may need a liver, kidney or heart transplant.
Liver transplantation aims to reduce the level of faulty TTR protein being synthesized, as the liver is where the protein is made. This approach is only indicated for treating patients with the hereditary form of the disease, which is caused by a mutation in the gene coding for the TTR protein.
ATTR-CM may also be associated with kidney impairment and protein in the urine due to the accumulation of misfolded TTR protein in the kidneys. A kidney transplant together with a liver transplant may sometimes be recommended.
A heart transplant may be considered in some patients with ATTR-CM and stage D, or the most advanced, heart failure with status 4 priority due to a lack of options for mechanical circulatory support.
Reviewed by Harshi Dhingra, M.D., on November 13, 2024. Updated March 24, 2025.